Imagine a healthcare system where digital is a determinant of health, and which respects the data acquired from and generated by patients and acknowledges their contribution to science.
In loving memory of Kawaldip Sehmi
Problem Statement
Data ties the individual and the system together as, without data, there is no such thing as personalised health. Eliasen (2021) suggests a new social contract that is respectful of not only the existing human rights perspective but also considers the rights associated with personal (and health) data. A proposed new social contract could encourage individuals to be active participants in, and take more ownership over, their health. This necessitates empowering patients and the general public to generate and share data responsibly and be empowered to use digital healthcare. While digitalisation plays an increasing role in the evolution of healthcare, there are substantial gaps in its implementation that need to be discussed and tackled.
Discussion Summary
Digital healthcare has become an important trend and future direction in healthcare, especially in resource-rich countries. This acceleration of digital health services and research has been documented by e.g., Beaverson in 2020, and then also in a Briefing to the European Parliament in 2021 (Negreiro 2021). A proposal of 2021 from the US White House's Office of Science and Technology Policy suggests the exploration and implementation of an “AI Bill of Rights”4, i.e. a comprehensive framework that provides a “good way to regulate [artificial intelligence]'s role in shaping a fair and equitable society without deciding what that society should look like, including how power should be balanced among individuals, corporations and the government” (Walsh, 2021).
The role of digitalisation in healthcare
Eric Topol suggests that digital health tools and applications will change healthcare in the near future and will also lead to more control by patients in terms of what happens to their health and data (2015), with this trend confirmed in a study by the Copenhagen Institute for Future Studies (2021). They find that “[h]ealth literacy in the future comes from turning personal health data into knowledge that helps individuals understand how their behaviour affects their health in the long term and how to act on this knowledge”. However, the accessibility of digital health tools is not uniform across communities around the world: these technologies tend to be expensive, a large portion of them are not available under open access, and they are otherwise resource-intensive. A report published in The Lancet points out that “the effect of digital transformations has been so pervasive that it might soon become a dominant prism through which we can understand and address health and wellbeing dynamics” (Kickbusch, Piselli, Agrawal et al. 2021).
Digital health technologies need to be aligned with country infrastructures and support services as also acknowledged by the WHO’s Global Strategy on Digital Health 2020-2025: “countries will adopt digital health in a way that is sustainable, respects their sovereignty, and best suits their culture and values, national health policy, vision, goals, health and well-being needs, and available resources” (WHO 2021). Gaps exist regarding local research and implementation of digital technologies which are currently mostly focused at global and aspirational levels. Groth, Nitzberg and Zehr (2019) state several challenges in the development and increased use of AI: vague or non-existent definitions of what AI is; very general strategies and vague objectives; scepticism; fragmentation; and the lack of computing capacities.
Member States of the World Health Organization (WHO) have adopted the Global Patient Safety Action Plan 2021–2030 (GPSAP 21-30) at the 2021 74th World Health Assembly, through its Decision WHA74(13) Agenda item 13.1. The GPSAP 21-30 has made room for patients to be owners and generators of patient safety data through the Framework for Action - The 7x5 Matrix and ensured a constant flow of patient and family generated data, information and knowledge to drive mitigation of risk, a reduction in levels of avoidable harm and improvements in the safety of care.
Further, each WHO Member States, when deciding in adopting the GPSAP 21-30 at the 74th World Health Assembly, resolved to work in collaboration with and include data from other stakeholders in addition to patient organisations in their society like their national civil society organisations, professional bodies, academic and research institutions, industry, and other relevant stakeholders to promote, prioritise and embed patient safety in all health policies and strategies.
Data and the person
With the explosive increase in the collection and processing of health-related personal data, the individual becomes their own biobank (Hafen 2019), storing large amounts of relevant data that make sense on their own and also as part of a big data environment. However, integration requires a bidirectional flow of data: from the patients to the data processors, and from the data processors back to the patients. It’s important to ensure that there is a feedback loop to patients on how their data is used to improve prevention, diagnosis, treatments, and services. This feedback loop will, in turn, empower them to advocate for wider data sharing.
Despite some progress with the implementation of the UN’s Sustainable Development Goals5, not everybody is accessing or is even aware of their basic human rights. However, the smart use of health data should push people and the data towards equity, and evidence-based patient advocacy also requires data. The WHO suggests in its Global Strategy on Digital Health 2020-2025 that “[d]igital health will be valued and adopted if it: is accessible and supports equitable and universal access to quality health services…”.
Closer cooperation across stakeholders including public institutions, companies and patients is essential. From the history of patient involvement in policy and research it’s clear that patient organisations are in an ideal position to facilitate cooperation. For example, involving patients in the innovation cycles is essential as they are the end users, and increasingly manage data. Some patient organisations have already begun to act as “honest data brokers'' Hafen (2019).
Patient data should thus be seen as the expression of the experience of the patients. Data can be cold, objectified and quantified, but it can also be filled with life if the focus is shifted back on the experience of the person represented by the data. Even in the case of simple metrics like blood pressure and weight - the question should also be asked: is the person living well and happy? Are they healthy?
Harnessing the power of patient data
The emergence and development of big data has affected life profoundly. Research topics are prioritised and healthcare systems are organised around different priorities now, and these priorities are increasingly linked to data. When assessing the impact of data, Hunter (2020) speaks about the “industrial revolution in biomedical research”. Patient constituencies are rightly concerned about how the misuse of data can be limited. At the same time, many patients are casual about the use of their data, and they need to understand what their data is used for and by whom. Referencing the Consultation Paper on Citizen-controlled health data sharing governance (Dantas, Mackiewicz, Tageo 2020), the European Patients’ Forum points out: “technology will always find its way if a culture of trust is settled first. Indeed, trust is seen as the cornerstone of this equation”6. A reasonable framework is needed to protect the integrity of patient data and minimise abuse, and to build trust. As argued below, there can be no personalised and humanised healthcare without the responsible use of data.
An important piece in the puzzle is the area of confidence and trust around responsible data sharing. The concept of ‘responsible data sharing’ is relatively new, and it is work in progress. One meta-analysis suggests that while there are some convergent basic principles around what responsible data sharing means, a consensus or generally accepted, universal guidelines have not yet emerged (Kalkman, Mostert, Gerlinger et al., 2019). The same authors suggest the following areas to consider when working towards responsible data sharing:
- societal benefits and value
- distribution of risks, benefits and burdens
- respect for individuals and groups; and
- public trust and engagement.
The IEEPO global community survey states a clear need by patient communities to have more influence in and control over how data are shared and used.
What are the biggest barriers for the patient community / your organisation when it comes to generating and/or using patient data? Please rank using a scale of 1 to 5 (1 = lowest barrier, 5 = biggest barrier)
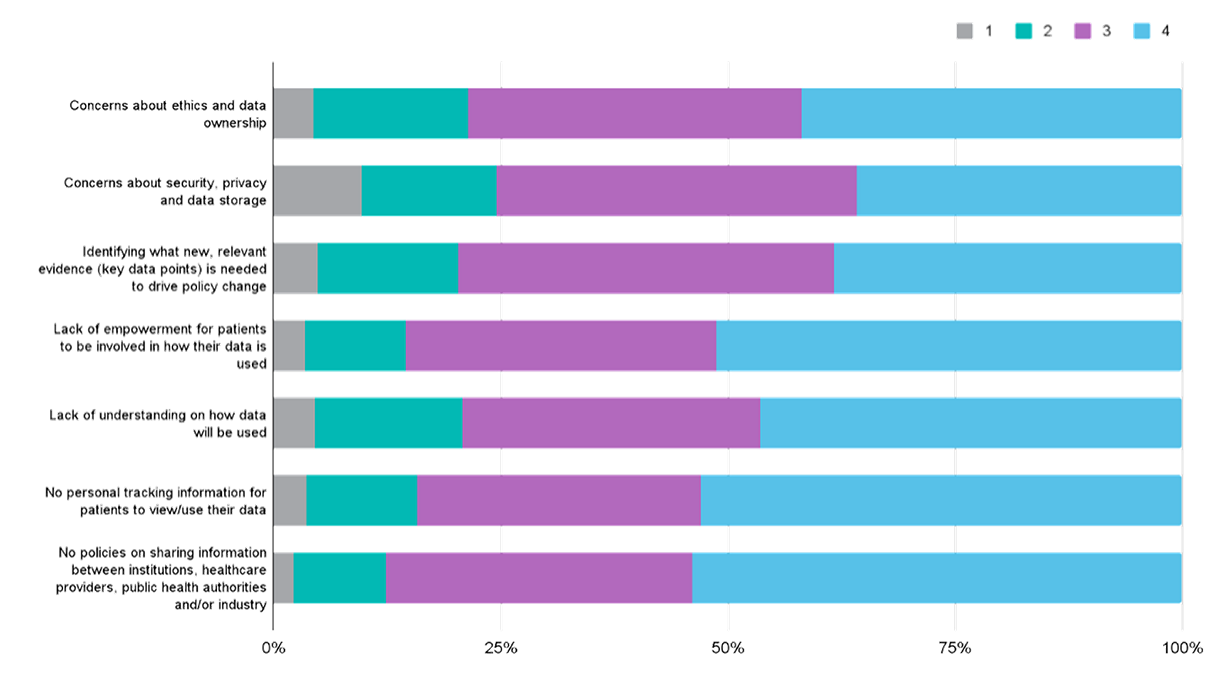
When asked about the biggest barriers for the patient community when it comes to generating and/or using patient data, the respondents saw the lack of clear policies on sharing data across different stakeholders as the most important factor that prevents them from participating.
Initiatives like Data Saves Lives7 try to deepen and enlarge the scope of responsible data sharing by discussing quality mutual information. This aspect is discussed in more detail in the chapter on “Humanising health literacy”.
One major concern remains: how does one collect, analyse and utilise real-world evidence? It’s believed that the key to the ultimate transformation lies there. Patients should be able to collect and mine information in a way that can help them make important healthcare decisions. Artificial intelligence (AI) can be a major asset in this field as AI can come up with meaningful explanations and evidence. Real-world patient data is key to incorporating real-world evidence in the body of scientific research consistently and meaningfully.
The IEEPO global community survey asked the patient communities specifically if they wanted to be part of this transformative process in terms of the deployment of artificial intelligence in healthcare.
To what extent do you agree with this statement? “I believe that Artificial Intelligence (AI) - based solutions can fundamentally change healthcare and that I as a patient representative can and should be part of this change.”
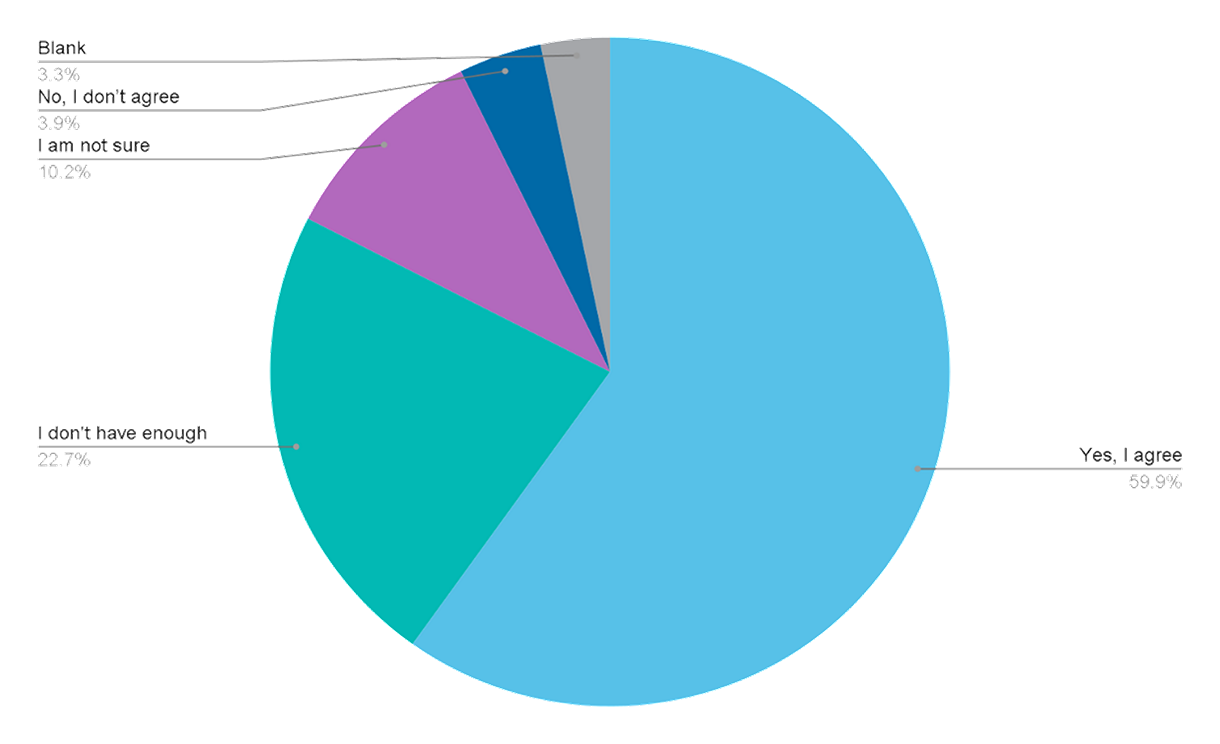
Almost 60% of the respondents agreed with the statement that patient community representatives should be part of the change brought about by the use of artificial intelligence.
This approach should lead to more recognition of and respect for traditional knowledge in health. This refers to anecdotal knowledge and experience that has not until now, been captured and described in the scientific paradigms (such as randomised clinical trials or studies). Traditional knowledge can also be turned into evidence with the help of big data and artificial intelligence (e.g., Mainenti 2019). Inclusion of traditional knowledge will also help reflect the diversity of cultures and societies as it allows consistent focus on certain aspects or parameters that are universal, such as biological features (Mukerji, Sagner 2019). It also allows the comparison of universal features of humans with variables that allow for diversity - genetic diseases and their treatment being a case in point. Real-world data will look different in different societies and cultures; therefore, contextualisation is key as it will change the utility of the data. In summary, traditional knowledge that now exists mostly as anecdotal evidence can and should be elevated to the level of reliable scientific evidence, thus creating a gateway to respectful and personalised healthcare.
Who controls the data?
Can it be argued that patients should control their own data? The jury is out on whether it is patient ownership or control that is needed. Patients need to be able to access their own data at any time and contribute with their experience. This is because patients are keen to see principles in place for the easy exchange of data across systems, so that the same information is given over and over as they move through the healthcare system. An environment needs to be created where data can flow freely across the systems without the need for costly and cumbersome interfaces and transformations. The proposed new European Health Data Space sets out to facilitate this objective: “A common European Health Data Space will promote better exchange and access to different types of health data (electronic health records, genomics data, data from patient registries etc.), not only to support healthcare delivery (so-called primary use of data) but also for health research and health policy making purposes (so-called secondary use of data)”8.
It is continuously stated that health data is the new oil: a natural resource that can be mined and used for many different profitable and beneficial purposes (Jacob, 2019). But is it really true? And are there perhaps opportunities to fast track some of the challenges? New ways need to be found to collect, analyse, and use data provided by patients voluntarily and involuntarily, whilst ensuring the data is unbiased. The risk of bias arises from the socio-cultural and economic backgrounds of the patient communities: the availability of digital means to collect data is not secured in many regions of the world. Wearables and smartphones are not available for patients in resource-constrained settings and collecting health data from chats and text messages is problematic.
However, it’s advised to start small and scale up. Social and community level projects can be designed to embed personalised healthcare through data. For companies, it may be a new strand in their corporate social responsibility efforts to dig deeper into health data to increase equity. In summary, patient data and input has a key role to play in
- shaping health policy
- participating in and shaping research and development
- patient reported outcomes
- patient quality of life reports; and
- quantitative and qualitative data from the patient community that can be used to advocate for improved policies around prevention, early diagnosis and holistic care (which goes beyond treatment), around disease management and treatment.
New data models can give individuals a better overview of their own health and can motivate them to take responsibility for their health. The system must, in return, provide the individual with tools to be able to collect and make sense of their own data. Big data and artificial intelligence should be used as a gateway to the integration and better understanding of traditional knowledge in health (Janska 2008) for it to become a gateway to personalised healthcare. This process needs consistent and easily accessible approaches in health literacy as discussed in the chapter “Humanising health literacy”.
Case Study: Enabling the Future of Cell & Gene Therapies through Non Proprietary Patient Owned Data Collection
Project background and objective
Though developing any therapy for a rare disease can be challenging, cell and gene therapies face additional hurdles both in the development phase and long af ter they are approved. During the discovery and development phase of rare disease therapies, the small patient populations that define these conditions can limit the understanding of a disease. The gold standard registration trial is often not possible in cell and gene therapy because of the lack of data and patients.
Real world evidence is one of the tools that can help address the challenges in both shaping the development path of an experimental therapy and also in providing insights into the safety and durability of these emerging therapies once they are approved. Understanding how a disease manifests itself, the way it progresses, and how measures of biomarkers of a given disease fluctuate and change over time is critical if a developer of a therapy hopes to provide proof that an experimental therapy is delivering m eaningful benefit to a patient.
Activity outputs and outcomes
By enabling rare patient communities to more easily gather, structure, and share critical data securely through a common platform in collaboration with researchers, drug developers, and clinic ians anywhere in the world, RARE X will accelerate diagnosis, disease understanding, and development of future treatments and cures across more than 9,500 rare diseases. One way to address the shared need for data is to eliminate the data silos that currently exist throughout the rare disease ecosystem. A federated data system is a meta database made up of connected databases. The databases remain independent and self contained, but they are transparently connected and can be queried together. Such a framework allows for the sharing of large datasets from around the world.
For patient communities, RARE-X supports their efforts to collect data, structure it, adhere to rigorous standards, and share it responsibly. Equally important, the organisation ensureS data that is developed with proper quality checks is validated and adheres to good clinical practice standards. For researchers and therapeutic developers, RARE X’s federated data platform gives access to the data they need to identify, develop, and trac k the impact of breakthrough treatments and cures.
RARE-X federated data platform is being developed with direct input from industry partners and regulators to ensure collaborators gather the data required and that the data adhere to regulatory standards. The organisation is initiating a series of demonstration projects in partnership with rare disease communities, biopharmaceutical companies, academic medical centres and other partners. The pilot programs will apply technology proven in other large scale public health and genomic data sharing initiatives to support the global needs of those developing treatments and caring for rare disease patients.
Further information: https://rare x.org/case studies/enabling the future of cell genetherapies through non proprietary patient owned data collection/
Calls to action
1. Patient communities should be empowered to generate data and share insights with both public health authorities and industry, in order to ‘have a seat at the table’. They can provide valuable data and patient insights to inform regulatory decisions, such as HTA processes, treatment reimbursement and work with industry to strengthen disease advocacy and medicines research and development (see case study above).
2. Build bridges and trust across disease areas and countries and work together with companies and with public institutions and civil society to share knowledge about responsible health data sharing.
3. Healthcare stakeholders, including industry, policymakers, healthcare professionals and technology companies must champion ‘responsible and inclusive innovation’ and enable equity of access in addition to privacy and transparency.
4. Create a guidance framework in partnership with the patient community, data experts, and policymakers that sets out best practice and ethical standards for how to generate, use and store patient data. The guidance framework should include transparency and greater understanding amongst patients, healthcare professionals/providers and policymakers around the value of patient data and how to use it safely and ethically.
5. Stakeholders should consider a code of conduct to support the goal of equitable access to artificial intelligence solutions, new and existing technology.
6. Advocate for patient involvement in co-creating solutions to humanise digital healthcare so they become innovators and instigators of change and transformation.
Link has been copied to clipboard
